AUthor: Jacob Boehm, MS
Edited By: James Sulzer, PhD
Reviewed by: Chanchal Agrawal, SPT
Correspondence:
jacob.boehm@austin.utexas.edu
ABSTRACT
The purpose of this review article is to consolidate research within the past 15 years on bimanual therapy for children (aged 8 months - 18 years) with hemiplegia. Bimanual therapy uses principles of motor learning, focusing on simultaneous use of both hands, to restore upper limb function in patients with motor deficits. A literature search was conducted using Google Scholar, and the results of 30 clinical trials were reviewed. A summary of study trends, including utilized outcome measures, is given. Then, a summary of findings for two main considerations of bimanual therapy: 1) patient factors, 2) methods of therapy implementation. General findings show intensive bimanual training is an effective form of therapy, especially over standard forms of care, but not over constraint induced movement therapy. Improvements following bimanual therapy were found for patients of all ages, severity of impairment, and motor pathway from brain to limb. Improvements reflected gains in bimanual ability and unimanual dexterity, evidenced by improvement in assessments such as the Assisting Hand Assessment and Jebsen-Taylor Test of Hand Function. A recent trend in studies is the use of neurophysiological measures, but the implications of results need to be further explored. Moreover, while bimanual therapy has been well studied for over two decades, there are still important questions to be answered.
1 INTRODUCTION
What makes intensive bimanual therapy effective and who can benefit? Since the 1990s, studies have shown that intense training leads to improved motor function for children with upper-limb motor deficits due to hemiplegia [1]. Training targets functional ability and active use of the affected limb(s), so children can independently perform tasks, like activities of daily living. Independence and functional ability are crucial to maintaining a high quality of life. In order to ensure the benefits of intensive bimanual therapy, clinical studies have compared various forms of bimanual therapy to other forms of upper-limb targeted therapy and observed the factors that contribute to outcomes of the therapy. Other forms of therapy include constraint-induced movement therapy (CIMT) and standard care (e.g. physio- and occupational therapy). Particular factors of interest include patient characteristics (e.g. diagnosis and age), frequency and amount of therapy, and characteristics of training (e.g. structured vs. unstructured).
In traditional CIMT, therapists restrain use of the unaffected limb by placing it in a restrictive cast, sling, or glove [2]–[4]. Patients then engage in unimanual, play or functional activities requiring active use of the affected limb. In accordance with motor learning principles, tasks become more difficult as practice progresses and improvements are noticed. Many studies have noted the functional gains made and maintained by CIMT and its related versions, but there is speculation as to its benefits over bimanual therapies, which do not require restraint of the affected limb and may more closely relate to how activities are performed in daily life.
As compared to CIMT, intensive bimanual therapy differs in two major aspects. It does not restrain use of the affected limb, and it focuses on simultaneous use of both limbs to perform an activity. The most popular means of delivering bimanual therapy is called hand-arm bimanual intensive training (HABIT) [5]. Like CIMT, HABIT engages patients in play and functional activities that involve gross and fine motor skills. Progressively difficult practice builds these bimanual skills, so patients can use them outside of the clinic. A couple of review papers have addressed HABIT and its efficacy as related to CIMT and patient characteristics. The review done by Gordon in 2011 [1] suggested that training intensity and repetition might effect outcomes more than characteristics of the training. It also suggested that bimanual training may result in more meaningful improvements marked by functional goal attainment and improved coordination of the two hands. However, CIMT may be better for patients with greater severity of impairment and particular deficits, like poor wrist supination. A second review done by Ouyang et al. in 2020 [6] looked at HABIT effects on upper-limb function. They found that HABIT improved upper limb function immediately after intervention, and the improvements were maintained at follow-up testing. They also reported improvement in selfcare and goal attainment.
Despite the years of evidence marking intensive bimanual training as an effective therapy, no review has yet addressed the underlying mechanisms of rehabilitation as they relate to bimanual training and patients. In particular, neurophysiological considerations, like motor pathways, have only recently been discussed in the literature. This review will update and summarize findings of clinical studies with respect to important factors of the patient population (those who stand to benefit most), treatment characteristics (best practices), and outcomes of intensive bimanual training (changes in neurophysiology and measures of ability).
2 MECHANISM OF ACTION
Intensive bimanual therapy aims to develop active use and functional ability of the upper-limbs by leveraging principles of motor learning [7]. In particular, intensive bimanual therapy uses practice specificity, i.e. use of the affected limb to perform a specific function during an activity. Through intensive, repetitive practice, the movements of the affected limb are “shaped” to fit the demands of an activity [8]. This is theorized to manifest physically as movement patterns generated by organized neural structures [8], [9]. These movement patterns work together to perform various motor skills. For children with neurological injury, there are multiple factors that can affect bimanual motor skills including (i) characteristics of movements, like symmetry, (ii) age and injury type, timing, and location, and (iii) reorganization of the corticospinal tract (CST) after injury.
Brain lesions affect motor areas leading to deficiency in motor planning and execution in the upper-limbs, which require training to overcome. Commonly, brain lesions result in hemiplegia, defined by muscular weakness or partial paralysis affecting one side of the body [10]. Due to weakness or paralysis, movements with particular characteristics are more difficult to make [11], [12]. Bimanual movements require activation of both hemispheres of the brain as opposed to more unilateral activation during unimanual movements [13]–[15]. Also, incongruent (asymmetric) movements require more processing than simple symmetric ones [15]. Since children with hemiplegia cannot fulfill these requirements in a typical manner, they often have poor bimanual coordination, preferring to move the upper limbs sequentially (unimanually) [16]. Bimanual training seeks to improve coordination by leveraging behavioral lateralization. Behavioral lateralization is the observed asymmetric use of the upper-limbs. In incongruent tasks, the non-dominant hand is used for assistance and stabilization while the dominant hand is used for fine motor manipulation [17], [18]. In HABIT protocol, the use of a patient’s affected hand is focused to mimic the typical use of a non-dominant hand in goal-oriented activities [5]. Use of the affected limb promotes growth of motor areas controlling the limb and in turn improves motor planning, execution, and coordination [19], [20].
Poor motor ability may also be attributed to interruption of typical brain development. Typically, motor pathways from the brain control the upper-limbs contralaterally [21], [22]. This cross organization occurs within the first six months of childhood development [23]. Before contralateral control fully develops, a phenomenon known as “mirror movements” occurs [24]. Mirror movements are unintentionally mirrored or coupled movements thought to be caused by “neural cross-talk” across hemispheres of the brain [13]. Mirror movements disappear after approximately the first 8 years of life and become undetectable by 10 years of age [24]. However, they have been shown to persist with childhood hemiplegia [25]. Some research suggests the persistence of mirror movements are caused by compromised integrity and myelination of the corpus callosum. The corpus callosum transmits inhibitory or excitatory commands between the hemispheres of the brain. Therefore, in brains with incomplete myelination or a compromised integrity, problems with inhibitory and excitatory motor commands can occur [26], [27]. Other hypotheses exist aside from corpus callosum immaturity or debility. These include abnormal interhemispheric inhibition between motor cortices, functional alteration of motor planning/execution, and abnormal persistence of the ipsilateral corticospinal tract [28]. Trouble decoupling movements has also been correlated with inattention, hyperactivity, and impulsivity [29]. Unfortunately, the extent to which the corpus collosum and other variables affect upper-limb movement are difficult to untangle and not well understood. Therefore, the question remains: can mirror movements and cross-talk be subjugated by intensive bimanual training practices or are they purely biological problems?
One insight to determining if bimanual therapy has effects related to the physical structure of motor pathways lies with the reorganization of the CST. Due to heterogeneity of disabilities, several organizational structures of the CST may reform (ipsilateral, contralateral, and bilateral). The reorganization of networks is dependent on the timing and location of injury [30]. Studies have correlated functional ability with reorganization of the CST. Most find that preservation of primarily contralateral pathways correlates with higher functionality. One in particular found that mirror movements are hardest to decouple when under ipsilateral control following contralateral damage [25]. This, however, does not mean that bimanual therapy is not as effective in children with other organizational structures. In a recent study, gains in upper-extremity function following bimanual therapy were found to be independent of CST reorganization for children with unilateral spastic cerebral palsy [31]. Regardless of organization, changes in CST structure and integrity correlating with these gains was evidenced by increases in activation and size of motor areas controlling the affected side as well as functional restoration of CST fibers [19], [20]. These changes in neurophysiology are hypothesized to be caused by the movement shaping practices of intensive bimanual therapy.
3 REVIEW METHODS
3.1 Literature Search Method
A search of the English language literature from 2008 to 2022 was performed using Google Scholar. Keywords used were pediatric, child*, bimanual, bilateral, therapy, rehabilitation, cerebral palsy, stroke, and traumatic brain injury. The first 200 results were scanned for clinical trials involving some form of intensive bimanual therapy for patients with neurologic injury aged 18 years and under. Out of the 200 results, 35 studies fit the criteria. From these 35 studies, 31 studies are included in the analysis of this review. Two of the four excluded studies were not fully accessible, one study had significant cross-reporting of data without significant difference in analysis, and one study primarily assessed the effects of botulinum toxin. A table summary of the studies may be found in the Appendix.
3.2 Study Comparison Method
Risk of bias was evaluated by one person with regard to random sequence generation, allocation concealment, blinding of participants and interventionists, blinding of outcome assessment, incomplete outcome data, selective reporting, and overall risk of bias. Each domain of bias was determined using the RoB 2 tool [32]. Statistical analysis was done using the Review Manager software, version 5.4.1, using standard mean differences and a random effects model.
4 TRENDS IN STUDY METHODOLOGY
4.1 Experimental Design
Of the 30 studies 20 were identified as randomized controlled trials (RCT), 2 as quasi-random trials, and 8 as within-subjects design.
4.1.1 Participants
All studies done included subjects diagnosed with a form of hemiplegia. Of these studies, 27 have subjects with unilateral CP and one tests subjects with bilateral CP. Particularly, 12 studies include subjects with spastic CP. One study considers subjects with hemispherectomy [33] and another also considers subjects with other causes of non-progressive central hemiplegia [3]. Ages of subjects mostly range from 4 years to 16 years of age, but one study included infants aged 8-16 months [2].
Other participant inclusion criteria specified manual ability classification system (MACS) scores of I-III and/or gross motor function classification system (GMFCS) scores of II-IV. Exclusion criteria included other health problems deemed to interfere with interventions, wrist and finger extension problems, inability to lift the affected arm, inability to grasp light objects with the affected hand, no discernable differences in Jebsen-Taylor Test of Hand Function (JTTHF) between limbs, poor cognitive ability assessed by level in mainstreamed school, inability to follow instruction, uncontrolled seizures, visual impairment deemed to interfere with intervention, severe muscle tone, recent botulinum toxin injections, and recent orthopedic surgery.
4.1.2 Interventions
Implementation of therapies did not vary from the core concept of intensive bimanual therapy, which engages patients in bimanual, goal-oriented, repetitive task practice. Activities are both functional and for play. A functional activity might be opening a drawer and manipulating the contents, while play activities can be card games or arts and crafts. Selected activities are age-appropriate and focus on particular deficits of upper-limb movement. As progress is made, difficulty of activities is also increased by requiring patients to perform tasks more quickly or more accurately. The most popular (n=20) method is HABIT and variations of HABIT. Variations of combine therapies of HABIT and other functional activities. Hand-arm bimanual intensive training including lower extremity (HABIT-ILE) includes exercises that require coordination of both upper- and lower-limbs [34]. Hand-arm bimanual intensive training tactile (HABIT-T) was proposed by one study to address sensory function and tactile discrimination [35]. Also, while many studies compare the effects of HABIT and CIMT or standard care, 2 studies combine CIMT and HABIT.
Therapy sessions were provided both in groups and individually. Many sessions were provided as part of camps lasting from 1 to 6 hours per day and ranging from 2-8 weeks. Some studies also reported use of a home or school program. The total dose (hours of therapy) range from 30 hours to 210 hours with the median at 90 hrs
4.2 Outcome Measures
Measures used to evaluate the efficacy and effects of bimanual therapy span from standardized scales of functional ability, including patient/parent questionnaires, to physical measures. This review will analyze results from all outcome measures but only compare data from the two most commonly used assessments stated below.
4.2.1 Scales of Functional Ability
Of the 31 included studies, over 11 different assessments evaluated changes in upper-limb function or ability. By far, the most common primary outcome measure was the Assisting Hand Assessment (AHA) used by 23 studies. The AHA is a test of use and performance of both hands during a variety of functional and play-based tasks. The second most common outcome measure was the Jebsen-Taylor Test of Hand Function (JTTHF), which assesses the time taken for a range of unimanual tasks to be completed. The third most common outcome measure was the Canadian Occupational Performance Measure (COPM), which is a patient or parent assessment of perceived performance of self-care, productivity, and leisure.
Other assessments, ordered by use in number of studies, are as follows: ABILHAND-Kids, Box and Block Test (BBT), Pediatric Evaluation of Disability (PEDI), Quality of Upper Extremity Skills Test (QUEST), Melbourne Assessment of Unilateral Upper Limb Function (MUUL), Goal Attainment Scale (GAS), Besta scale, and Pediatric Upper-extremity Motor Activity Log-Revised (PMAL-R). Some studies include task-specific performance measures, such as the number of items discriminated during a tactile stimulation test.
4.2.2 Physical Measures
A few studies consider physical measures computed from sensor or imaging data. One study includes accelerometry data [4], and two others include kinematic data from motion capture [16], [36]. More recently, neurophysiologic signals were acquired by functional Magnetic Resonance Imaging (fMRI), Diffusion Tensor Imaging (DTI), and Transcranial Magnetic Stimulation (TMS). These signals are processed to indicate white matter integrity by fractional anisotropy (FA) or mean diffusivity (MD), size of motor maps, or a computed radiological score. The radiologic score covers number of affected lobes, volume and type of white matter injury, extent of gray matter damage, and major white matter tract injury [37]. Results of physical measures were all correlated with a particular therapy method or other outcome measures. In particular, one study compared white matter integrity and CST laterality to AHA and JTTHF [38].
5 STUDY FINDINGS
For all studies involving some form of bimanual therapy, statistically significant improved function, ability, or physiology was found.
5.1 Treatment Factors
5.1.1 vs Standard Care
As compared to standard care, intensive bimanual therapy was found to improve functional ability of the upper extremities for multiple outcome measures [19], [39]–[41]. Results from a large, multi-site clinical trial showed significant improvements in function of the upper-limb for children who underwent intensive bimanual therapy, whereas children receiving standard care showed minimal or no change [40]. Improvements were evidenced by increases in QUEST scores and the Besta scale. These improvements were maintained at 3- and 6-month follow-up tests. Another study showed HABIT correlates greater improvements in perceived performance and satisfaction (COPM) as well as functional skills and reduced caregiver assistance (PEDI) as compared to standard care [39].
One study showed functional restoration of the CST originating from both non-lesioned and lesioned hemispheres to coincide with HABIT-ILE [19]. Fractional Anisotropy (FA) significantly increased and mean diffusivity (MD) significantly decreased for subjects in the HABIT-ILE group as compared to subjects in a control group receiving standard care. Both changes in FA and MD correlated with improvements manual ability and perceived manual ability indicated by increases in BBT and ABILHAND-Kids scores. Overall, evidence points toward intensive bimanual therapy to achieve greater functional and physical gains than just standard care.
5.1.2 vs CIMT
As compared to CIMT, mixed results were found for outcome measures, potentially due to task-specificity, but overall improvement did not statistically differ between methods of therapy [2]–[4], [40], [42]–[47]. In a study done on infants, both modified CIMT and bimanual therapy correlated equally with improvements in a mini-AHA, which indicates improved use of the affected hand [2]. However, this study did not include a control group, so it is questionable if CIMT or bimanual therapy are beneficial to infant populations with CP. Five studies showed similar gains made by modified-CIMT and intensive bimanual therapy in AHA scores [3], [4], [31], [43], [46]. However, for one of these studies greater gains were made in MUUL for the affected limb for modified-CIMT [3], and for another, only the bimanual therapy group maintained improvements in AHA after 26 weeks [46]. A summary of compared results for bimanual ability gains can be found in Fig. 1. Comparable gains were also made in unimanual dexterity, evidenced by decreases in JTTHF scores, for patients in HABIT and CIMT groups (Fig. 2). However, in general CIMT tends to improve unimanual dexterity of the affected limb more. Several studies suggest that this outcome is due to specificity of practice. Intensive bimanual therapy trains use of both limbs and the AHA tests use of both limbs together. Constraint induced movement therapy trains use of the affected limb through unimanual tasks and JTTHF is a unimanual test.
5.1.3 Location and Dose
Studies addressing the variables of the prescribed therapy, such as dosage, location of administration, and modification of activities, did not tend to find significant differences in improvement across variables[48]–[51]. In a school-based setting, patients doing HABIT improved in both AHA and QUEST scores, which were maintained at 6-month follow-up [43]. Another study held therapy sessions at a magic themed camp at a hospital facility and reported significant improvement in AHA scores [27]. A third study assessed the efficacy of a caregiver-directed, home-based program of HABIT and found significant improvement in BBT and COPM scores as compared to a control group [52]. However, the HABIT group did not have any significant improvement in AHA scores.
Two studies addressed the dose of bimanual therapy. One small study with 9 subjects in each group assessed 45 hours of bimanual therapy versus 90 hours of bimanual therapy over a 3 week period [48]. Both groups had significant improvements in both AHA and JTTHF scores without differences between groups. The other study compared one large group of 32 subjects receiving 90 hours of therapy to a smaller group of 9 subjects receiving only 30 hours of therapy [51]. Findings showed no between- group differences for bimanual therapy doses in measures of AHA, JTTFH, or COPM. Although, the low dose group did not make significant within-group gains on any upper-limb motor outcome, gains in occupational performance were clinically meaningful. It should be noted that both of these studies have small sample sizes for low dose groups, so more research need be done to determine proper dosing of bimanual therapy.
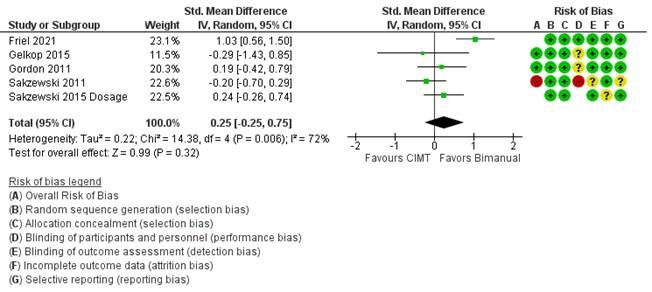
Figure 1. Comparison of changes in bimanual ability marked by Assisting Hand Assessment (AHA) scores for bimanual therapy vs CIMT. Studies are organized by overall risk of bias shown in the Risk of Bias A column and weighted by number of subjects. For risk of bias, green indicates low risk, yellow indicates unclear risk, and red indicates high risk. A positive standard mean difference favors bimanual therapy over CIMT for improvement in bimanual ability. Only one study (Friel 2021) clearly indicated favor of bimanual therapy over CIMT, and overall results lean in favor of bimanual therapy for improving bimanual ability, but are not statistically significant.
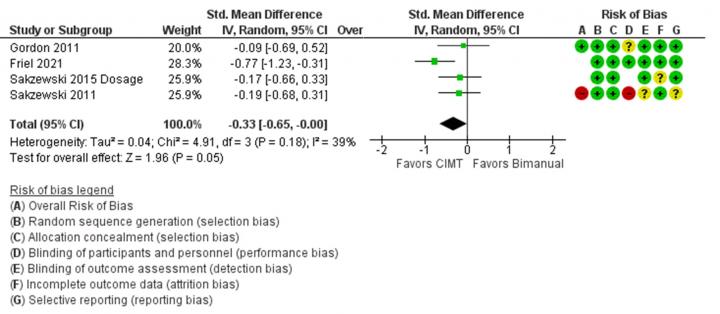
Figure 2. Comparison of changes in unimanual dexterity marked by Jebsen-Taylor Test of Hand Function (JTTHF) scores for bimanual therapy vs CIMT. Studies are organized by overall risk of bias shown in the Risk of Bias A column and weighted by number of subjects. For risk of bias, green indicates low risk, yellow indicates unclear risk, and red indicates high risk. A negative standard mean difference favors CIMT over bimanual therapy for improvement in unimanual dexterity. Only one study (Friel 2021) clearly indicated favor of CIMT over bimanual therapy, and overall results are just statistically significant in favor of CIMT.
5.2 Patient Factors
5.2.1 Age
Several studies addressed the variability of patient age on interventional outcome. Intensive bimanual therapy has been studied for patients of all ages. In this review, ages range from 8 months to 18 years. Some literature suggests that gains in upper-limb ability following intensive bimanual therapy might be associated with age of participants. However, multiple studies found that age did not correlate with any treatment outcome [3], [4], [31]. No other studies in this review reported correlation of age with outcomes. The most probable reason for age not having an effect on gains made through intensive bimanual therapy is the use of “age-appropriate” and progressively difficult activities. Thus, age may not be an important factor regarding bimanual therapy intervention.
5.2.2 Severity of Impairment
Another important factor regarding patients are the characteristics of impairment. Do particular populations stand to benefit from bimanual therapy more? One study addressed the timing of therapy relative to injury but found no significant differences in improvement between the groups with regard to increases in activation and size of motor areas controlling the affected limb [53]. A few studies suggest that more severely impaired patients stand to gain more from bimanual therapy [3], [31]. One found that more-disabled children showed greater improvement than less-disabled ones on the AHA [3]. This, however, should be considered with caution due to only one study reporting this finding and due to patient recruitment trends. Patients have been excluded from studies if the severity of impairment was too great to fully participate in activities. Also, HABIT-ILE was tested for children with bilateral CP [41]. Findings from this study show significant improvements in function of the more affected limbs from BBT and JTTHF. Patients also reported less perceived difficulty to perform tasks from ABILHAND-Kids assessment and improved self-care from PEDI.
5.2.3 CST Organization
As mentioned in Section 2, it is thought that reorganization of the CST leads to different levels of impairment based on the lateralization (i.e. ipsilateral, contralateral, or bilateral control of the upper-limbs). It follows that CST organization might play a role in functional gains made by patients undergoing intensive bimanual therapy. Five studies reported the use of imaging techniques to determine CST organization and laterality [19], [20], [31], [38], [54]. One study reported a shift in lateralization coinciding with increased activation in the affected hemisphere as measured by fMRI [38]. Two studies reported that CST organization, determined by TMS, was independent of improvements made in AHA and JTTHF scores [31], [54]. One of these studies also found no correlation between improvements in BBT, COPM, or PEDI with CST organization [31]. A fourth study also found that CST organization, determined by TMS, was independent of increases in activation and size of motor areas controlling the affected limb [20]. An aspect of these studies to note is the difference in imaging techniques used to define the CST organization, which may have discrepancies between techniques [55]. Nonetheless, all studies found no dependence of CST organization with gains made by subjects, thereby suggesting that intensive bimanual therapy can benefit patients of all types. The reason behind this could very well be the neural networks formed during bimanual practice which are able to generate the specifically practiced movements regardless of CST structure.
6 DISCUSSION
6.1 Takeaways for Clinicians
By all accounts, studies in the past 15 years have shown intensive bimanual therapy is effective for improving bimanual function for children with hemiplegia that have upper-limb motor deficits. The benefits of bimanual therapy over CIMT or vice versa are only barely statistically significant for unimanual dexterity as measured by the JTTHF when compared across studies [3], [4], [31], [43], [46]. However, the benefits of intensive bimanual therapy over standard physio- and occupational therapy are quite apparent [19], [39]–[41].
The proper dose of bimanual therapy is still unknown. Most studies have patients receive 90 hours of therapy. There are studies that show lower doses down to only 30 hours can make similar improvements, but these studies are limited by small sample sizes [48], [51]. A large variety of children with hemiplegia stand to benefit from bimanual therapy. Gains are shown to be independent of age [3], [4], [31], timing of therapy relative to injury [53], and organization/lateralization of the corticospinal tract [27]–[29], [51]. Patients with more severe impairment, such that it does not interfere with administration of bimanual therapy, might benefit from bimanual therapy more. However, this has only been shown in a few studies [31], [3]. This also presents a limitation to bimanual therapy. Children with too severe of impairments cannot improve upper-limb function because they simply cannot participate, and children with more mild impairment are less likely to participate in bimanual therapy. Bimanual therapy has shown to be effective in both clinics, school environments, and at home; however, most studies took place in a clinical environment. Therefore, caution with procedures for school or home programs need be taken.
6.2 Takeaways for Researchers
Bimanual therapy is a well-studied topic with a lot of reported data. However, there are still questions that remain to make it truly effective for all patients. In many studies, even if the group showed improvements, some subjects did not improve. This was not correlated to any patient factors or methods of therapy implementation. More exploration into patient factors and specifics of practice may uncover the reasons behind individual improvement. Given the amount of studies and subjects participating in studies, there is plenty of data regarding patients, methods of therapy, and outcomes. However, due to the inaccessibility of that data, heterogeneity in reporting results, and heterogeneity in outcome measures, comparison is stifled.
Implications of neurophysiological measures and the changes in these measures due to intensive bimanual therapy still need more research. Insight to best practices might be attained by relating the characteristics of movement, engagement, and other factors of practice during therapy sessions to motor areas of the brain. These measures may also help determine how to mechanically influence the restoration and development of the central nervous system.
References
[1] A. M. Gordon, “To constrain or not to constrain, and other stories of intensive upper extremity training for children with unilateral cerebral palsy,” Dev. Med. Child Neurol., vol. 53, no. SUPPL.4, pp. 56–61, 2011, doi: 10.1111/j.1469-8749.2011.04066.x.
[2] R. Chamudot, S. Parush, A. Rigbi, R. Horovitz, and V. Gross-Tsur, “Effectiveness of modified constraint-induced movement therapy compared with bimanual therapy home programs for infants with hemiplegia: A randomized controlled trial,” Am. J. Occup. Ther., vol. 72, no. 6, 2018, doi: 10.5014/AJOT.2018.025981.
[3] W. Deppe, K. Thuemmler, J. Fleischer, C. Berger, S. Meyer, and B. Wiedemann, “Modified constraint-induced movement therapy versus intensive bimanual training for children with hemiplegia-a randomized controlled trial,” Clin. Rehabil., vol. 27, no. 10, pp. 909–920, 2013, doi: 10.1177/0269215513483764.
[4] A. M. Gordon et al., “Bimanual training and constraint-induced movement therapy in children with hemiplegic cerebral palsy: A randomized trial,” Neurorehabil. Neural Repair, vol. 25, no. 8, pp. 692–702, 2011, doi: 10.1177/1545968311402508.
[5] J. Charles and A. M. Gordon, “Development of hand-arm bimanual intensive training (HABIT) for improving bimanual coordination in children with hemiplegic cerebral palsy,” Dev. Med. Child Neurol., vol. 48, no. 11, pp. 931–936, 2006, doi: 10.1017/S0012162206002039.
[6] R. G. Ouyang, C. N. Yang, Y. L. Qu, M. P. Koduri, and C. W. Chien, “Effectiveness of hand-arm bimanual intensive training on upper extremity function in children with cerebral palsy: A systematic review,” Eur. J. Paediatr. Neurol., vol. 25, pp. 17–28, 2020, doi: 10.1016/j.ejpn.2019.12.017.
[7] A. M. Gordon and R. Magill, “Motor learning: application of principles to pediatric rehabilitation,” Campbell’s Phys. Ther. Child. Expert Consult., p. 78, 2016.
[8] E. J. Plautz, G. W. Milliken, and R. J. Nudo, “Effects of repetitive motor training on movement representations in adult squirrel monkeys: Role of use versus learning,” Neurobiol. Learn. Mem., vol. 74, no. 1, pp. 27–55, 2000, doi: 10.1006/nlme.1999.3934.
[9] M. L. Latash and J. G. Anson, “What are ‘normal movements’ in atypical populations?,” Behav. Brain Sci., vol. 19, no. 1, pp. 55–68, 1996, doi: DOI: 10.1017/S0140525X00041467.
[10] M. Oskoui and M. I. Shevell, “Profile of pediatric hemiparesis,” J. Child Neurol., vol. 20, no. 6, pp. 471–476, 2005, doi: 10.1177/088307380502000601.
[11] S. Y. Schaefer, K. Y. Haaland, and R. L. Sainburg, “Hemispheric specialization and functional impact of ipsilesional deficits in movement coordination and accuracy,” Neuropsychologia, vol. 47, no. 13, pp. 2953–2966, 2009, doi: 10.1016/j.neuropsychologia.2009.06.025.
[12] S. Mani, P. K. Mutha, A. Przybyla, K. Y. Haaland, D. C. Good, and R. L. Sainburg, “Contralesional motor deficits after unilateral stroke reflect hemisphere-specific control mechanisms,” Brain, vol. 136, no. 4, pp. 1288–1303, 2013, doi: 10.1093/brain/aws283.
[13] S. P. Swinnen and N. Wenderoth, “Two hands, one brain: Cognitive neuroscience of bimanual skill,” Trends Cogn. Sci., vol. 8, no. 1, pp. 18–25, 2004, doi: 10.1016/j.tics.2003.10.017.
[14] S. Koeneke, K. Lutz, T. Wüstenberg, and L. Jäncke, “Bimanual versus unimanual coordination: What makes the difference?,” Neuroimage, vol. 22, no. 3, pp. 1336–1350, 2004, doi: 10.1016/j.neuroimage.2004.03.012.
[15] G. W. Goerres, M. Samuel, I. H. Jenkins, and D. J. Brooks, “Cerebral control of unimanual and bimanual movements,” Neuroreport, vol. 9, no. 16, pp. 3631–3638, 1998.
[16] Y. C. Hung, L. Casertano, A. Hillman, and A. M. Gordon, “The effect of intensive bimanual training on coordination of the hands in children with congenital hemiplegia,” Res. Dev. Disabil., vol. 32, no. 6, pp. 2724–2731, 2011, doi: 10.1016/j.ridd.2011.05.038.
[17] Y. Guiard, “Asymmetric division of labor in human skilled bimanual action: The kinematic chain as a model,” J. Mot. Behav., vol. 19, no. 4, pp. 486–517, 1987, doi: 10.1080/00222895.1987.10735426.
[18] P. J. Bryden, “The influence of M. P. Bryden’s work on lateralization of motor skill: Is the preferred hand selected for and better at tasks requiring a high degree of skill?,” Laterality, vol. 21, no. 4–6, pp. 312–328, 2016, doi: 10.1080/1357650X.2015.1099661.
[19] Y. Bleyenheuft et al., “Motor Skill Training May Restore Impaired Corticospinal Tract Fibers in Children With Cerebral Palsy,” Neurorehabil. Neural Repair, vol. 34, no. 6, pp. 533–546, 2020, doi: 10.1177/1545968320918841.
[20] Y. Bleyenheuft et al., “Capturing neuroplastic changes after bimanual intensive rehabilitation in children with unilateral spastic cerebral palsy: A combined DTI, TMS and fMRI pilot study,” Res. Dev. Disabil., vol. 43–44, pp. 136–149, 2015, doi: 10.1016/j.ridd.2015.06.014.
[21] H. G. J. M. Kuypers, “The Descending Pathways to the Spinal Cord, their Anatomy and Function,” Prog. Brain Res., vol. 11, pp. 178–202, 1964.
[22] J. Brinkman and H. G. J. M. Kuypers, “Splitbrain monkeys: Cerebral control of ipsilateral and contralateral arm, hand, and finger movements,” Science (80-. )., vol. 176, no. 4034, pp. 536–539, 1972, doi: 10.1126/science.176.4034.536.
[23] J. A. Eyre et al., “Is hemiplegic cerebral palsy equivalent to amblyopia of the corticospinal system?,” Ann. Neurol., vol. 62, no. 5, pp. 493–503, 2007, doi: 10.1002/ana.21108.
[24] P. Leinen, S. Vieluf, D. Kennedy, G. Aschersleben, C. H. Shea, and S. Panzer, “Life span changes: Performing a continuous 1:2 bimanual coordination task,” Hum. Mov. Sci., vol. 46, pp. 209–220, 2016, doi: 10.1016/j.humov.2016.01.004.
[25] B. T. Woods and H.-L. Teuber, “Mirror movements after childhood hemiparesis,” Neurology, vol. 28, no. 11, pp. 1152 LP – 1152, Nov. 1978, doi: 10.1212/WNL.28.11.1152.
[26] B. U. Meyer, S. Röricht, H. G. Von Einsiedel, F. Kruggel, and A. Weindl, “Inhibitory and excitatory interhemispheric transfers between motor cortical areas in normal humans and patients with abnormalities of the corpus callosum,” Brain, vol. 118, no. 2, pp. 429–440, 1995, doi: 10.1093/brain/118.2.429.
[27] D. Green et al., “A multi-site study of functional outcomes following a themed approach to hand-arm bimanual intensive therapy for children with hemiplegia,” Dev. Med. Child Neurol., vol. 55, no. 6, pp. 527–533, 2013, doi: 10.1111/dmcn.12113.
[28] C. Galléa, T. Popa, S. Billot, A. Méneret, C. Depienne, and E. Roze, “Congenital mirror movements: A clue to understanding bimanual motor control,” J. Neurol., vol. 258, no. 11, pp. 1911–1919, 2011, doi: 10.1007/s00415-011-6107-9.
[29] D. J. Serrien, M. M. Sovijärvi-Spapé, and G. Rana, “Developmental changes in motor control: Insights from bimanual coordination,” Dev. Psychol., vol. 50, no. 1, pp. 316–323, 2014.
[30] A. M. Gordon, “Impaired Voluntary Movement Control and Its Rehabilitation in Cerebral Palsy BT - Progress in Motor Control: Theories and Translations,” J. Laczko and M. L. Latash, Eds. Cham: Springer International Publishing, 2016, pp. 291–311.
[31] K. M. Friel et al., “Improvements in Upper Extremity Function Following Intensive Training Are Independent of Corticospinal Tract Organization in Children With Unilateral Spastic Cerebral Palsy: A Clinical Randomized Trial,” Front. Neurol., vol. 12, no. May, pp. 1–15, 2021, doi: 10.3389/fneur.2021.660780.
[32] J. A. C. Sterne et al., “RoB 2: A revised tool for assessing risk of bias in randomised trials,” BMJ, vol. 366, pp. 1–8, 2019, doi: 10.1136/bmj.l4898.
[33] M. T. Robert et al., “Intensive Bimanual Intervention for Children Who Have Undergone Hemispherectomy: A Pilot Study,” Pediatr. Phys. Ther., vol. 33, no. 3, pp. 120–127, 2021, doi: 10.1097/PEP.0000000000000804.
[34] Y. Bleyenheuft and A. M. Gordon, “Hand-arm bimanual intensive therapy including lower extremities (HABIT-ILE) for children with cerebral palsy,” Phys. Occup. Ther. Pediatr., vol. 34, no. 4, pp. 390–403, 2014.
[35] H. C. Kuo, A. M. Gordon, A. Henrionnet, S. Hautfenne, K. M. Friel, and Y. Bleyenheuft, “The effects of intensive bimanual training with and without tactile training on tactile function in children with unilateral spastic cerebral palsy: A pilot study,” Res. Dev. Disabil., vol. 49–50, pp. 129–139, 2016, doi: 10.1016/j.ridd.2015.11.024.
[36] Y. C. Hung, A. Spingarn, K. M. Friel, and A. M. Gordon, “Intensive Unimanual Training Leads to Better Reaching and Head Control than Bimanual Training in Children with Unilateral Cerebral Palsy,” Phys. Occup. Ther. Pediatr., vol. 40, no. 5, pp. 491–505, 2020, doi: 10.1080/01942638.2020.1712513.
[37] S. I. Shiran et al., “MRI-based radiologic scoring system for extent of brain injury in children with hemiplegia,” Am. J. Neuroradiol., vol. 35, no. 12, pp. 2388–2396, 2014, doi: 10.3174/ajnr.A3950.
[38] M. Weinstein et al., “Brain Plasticity following Intensive Bimanual Therapy in Children with Hemiparesis: Preliminary Evidence,” Neural Plast., vol. 2015, 2015, doi: 10.1155/2015/798481.
[39] P. R. P. Figueiredo et al., “Hand–arm bimanual intensive therapy and daily functioning of children with bilateral cerebral palsy: a randomized controlled trial,” Dev. Med. Child Neurol., vol. 62, no. 11, pp. 1274–1282, 2020, doi: 10.1111/dmcn.14630.
[40] E. Fedrizzi et al., “Unimanual and bimanual intensive training in children with hemiplegic cerebral palsy and persistence in time of hand function improvement: 6-month follow-up results of a multisite clinical trial,” J. Child Neurol., vol. 28, no. 2, pp. 161–175, 2013, doi: 10.1177/0883073812443004.
[41] Y. Bleyenheuft et al., “Intensive upper- and lower-extremity training for children with bilateral cerebral palsy: a quasi-randomized trial,” Dev. Med. Child Neurol., vol. 59, no. 6, pp. 625–633, 2017, doi: 10.1111/dmcn.13379.
[42] L. Sakzewski, S. Carlon, N. Shields, J. Ziviani, R. S. Ware, and R. N. Boyd, “Impact of intensive upper limb rehabilitation on quality of life: A randomized trial in children with unilateral cerebral palsy,” Dev. Med. Child Neurol., vol. 54, no. 5, pp. 415–423, 2012, doi: 10.1111/j.1469-8749.2012.04272.x.
[43] N. Gelkop et al., “Efficacy of constraint-induced movement therapy and bimanual training in children with hemiplegic cerebral palsy in an educational setting,” Phys. Occup. Ther. Pediatr., vol. 35, no. 1, pp. 24–39, 2015, doi: 10.3109/01942638.2014.925027.
[44] P. Facchin et al., “Multisite trial comparing the efficacy of constraint-induced movement therapy with that of bimanual intensive training in children with hemiplegic cerebral palsy: Postintervention results,” Am. J. Phys. Med. Rehabil., vol. 90, no. 7, pp. 539–553, 2011, doi: 10.1097/PHM.0b013e3182247076.
[45] A. M. Gordon, A. Chinnan, S. Gill, E. Petra, Y. Hung, and J. Charles, “Both constraint‐induced movement therapy and bimanual training lead to improved performance of upper extremity function in children with hemiplegia,” Dev. Med. Child Neurol., vol. 50, no. 12, pp. 957–958, 2008.
[46] L. Sakzewski, J. Ziviani, D. F. Abbott, R. A. L. Macdonell, G. D. Jackson, and R. N. Boyd, “Randomized trial of constraint-induced movement therapy and bimanual training on activity outcomes for children with congenital hemiplegia,” Dev. Med. Child Neurol., vol. 53, no. 4, pp. 313–320, 2011, doi: 10.1111/j.1469-8749.2010.03859.x.
[47] H. Bingöl and M. K. Günel, “Comparing the effects of modified constraint-induced movement therapy and bimanual training in children with hemiplegic cerebral palsy mainstreamed in regular school: A randomized controlled study,” Arch. Pediatr., vol. 29, no. 2, pp. 105–115, 2022, doi: 10.1016/j.arcped.2021.11.017.
[48] M. B. Brandão et al., “Does Dosage Matter? A Pilot Study of Hand-Arm Bimanual Intensive Training (HABIT) Dose and Dosing Schedule in Children with Unilateral Cerebral Palsy,” Phys. Occup. Ther. Pediatr., vol. 38, no. 3, pp. 227–242, 2018, doi: 10.1080/01942638.2017.1407014.
[49] C. L. E. Peper, E. C. P. Van Loon, A. Van De Rijt, A. Salverda, and A. A. Van Kuijk, “Bimanual training for children with cerebral palsy: Exploring the effects of Lissajous-based computer gaming,” Dev. Neurorehabil., vol. 16, no. 4, pp. 255–265, 2013, doi: 10.3109/17518423.2012.760116.
[50] M. Cohen-Holzer, G. Sorek, S. Schless, J. Kerem, and M. Katz-Leurer, “The influence of a constraint and bimanual training program using a variety of modalities, on upper extremity functions and gait parameters among children with hemiparetic cerebral palsy: A case series,” Phys. Occup. Ther. Pediatr., vol. 36, no. 1, pp. 17–27, 2016, doi: 10.3109/01942638.2014.990549.
[51] L. Sakzewski, K. Provan, J. Ziviani, and R. N. Boyd, “Comparison of dosage of intensive upper limb therapy for children with unilateral cerebral palsy: How big should the therapy pill be?,” Res. Dev. Disabil., vol. 37, pp. 9–16, 2015, doi: 10.1016/j.ridd.2014.10.050.
[52] C. L. Ferre, M. Brandão, B. Surana, A. P. Dew, N. G. Moreau, and A. M. Gordon, “Caregiver-directed home-based intensive bimanual training in young children with unilateral spastic cerebral palsy: a randomized trial,” Dev. Med. Child Neurol., vol. 59, no. 5, pp. 497–504, 2017, doi: 10.1111/dmcn.13330.
[53] Y. Bleyenheuft, C. Arnould, M. B. Brandao, C. Bleyenheuft, and A. M. Gordon, “Hand and Arm Bimanual Intensive Therapy Including Lower Extremity (HABIT-ILE) in Children With Unilateral Spastic Cerebral Palsy: A Randomized Trial,” Neurorehabil. Neural Repair, vol. 29, no. 7, pp. 645–657, 2015, doi: 10.1177/1545968314562109.
[54] A. R. P. Smorenburg et al., “Does Corticospinal Tract Connectivity Influence the Response to Intensive Bimanual Therapy in Children with Unilateral Cerebral Palsy?,” Neurorehabil. Neural Repair, vol. 31, no. 3, pp. 250–260, 2017, doi: 10.1177/1545968316675427.
[55] M. Weinstein et al., “Understanding the relationship between brain and upper limb function in children with unilateral motor impairments: a multimodal approach,” Eur. J. Paediatr. Neurol., vol. 22, no. 1, pp. 143–154, 2018.
[56] Y. C. Hung, M. B. Brandão, and A. M. Gordon, “Structured skill practice during intensive bimanual training leads to better trunk and arm control than unstructured practice in children with unilateral spastic cerebral palsy,” Res. Dev. Disabil., vol. 60, pp. 65–76, 2017, doi: 10.1016/j.ridd.2016.11.012.
[57] L. Sakzewski et al., “Randomized comparison trial of density and context of upper limb intensive group versus individualized occupational therapy for children with unilateral cerebral palsy,” Dev. Med. Child Neurol., vol. 57, no. 6, pp. 539–547, 2015, doi: 10.1111/dmcn.12702.
[58] P. B. Aarts, P. H. Jongerius, Y. A. Geerdink, J. van Limbeek, and A. C. Geurts, “Modified Constraint-Induced Movement Therapy combined with Bimanual Training (mCIMT-BiT) in children with unilateral spastic cerebral palsy: How are improvements in arm-hand use established?,” Res. Dev. Disabil., vol. 32, no. 1, pp. 271–279, 2010, doi: 10.1016/j.ridd.2010.10.008.
[59] K. M. Friel et al., “Skilled Bimanual Training Drives Motor Cortex Plasticity in Children with Unilateral Cerebral Palsy,” Neurorehabil. Neural Repair, vol. 30, no. 9, pp. 834–844, 2016, doi: 10.1177/1545968315625838.
[60] M. Schertz et al., “Imaging Predictors of Improvement from a Motor Learning-Based Intervention for Children with Unilateral Cerebral Palsy,” Neurorehabil. Neural Repair, vol. 30, no. 7, pp. 647–660, 2016, doi: 10.1177/1545968315613446.
[61] T. Levy, A.-A. Field, D. Humpl, and H. Atkinson, “Intense Bimanual Therapy in Childhood Hemiparesis: Translating Research Into Practice & Parent Reported Outcomes,” Arch. Phys. Med. Rehabil., vol. 99, no. 12, p. e205, 2018.
